 CIMS Global founder Dr. Tai Xie, an industry pioneer focused on reshaping the future of clinical trials through the convergence of EDC and IWRS, statistical modeling and machine learning, will be speaking at DIA China on May 22, 2019.
CIMS Global founder Dr. Tai Xie, an industry pioneer focused on reshaping the future of clinical trials through the convergence of EDC and IWRS, statistical modeling and machine learning, will be speaking at DIA China on May 22, 2019.
Dr. Xie’s talk, titled Use of EHR Data in Clinical Trials (Understanding FDA’s Recent Guidance), will discuss how the FDA has outlined the path for using EHR/EMR data in clinical trials, along with the opportunities and the challenges.
Overview – Use of EHR Data in Clinical Trials
A decade ago, clinical trials were “paper”-based (i.e. paper data capture or PDC) meaning the patient data were recorded on paper Case Report Form (CRF). The paper CRFs were sent to data management center for double-key data entry. One of the problems in PDC was transcription errors. With the rapid development of internet technology, the old PDC has been replaced with Electronic Data Capture (EDC). Nowadays, nearly 90% clinical trials are managed by using an EDC system. The initial idea of EDC was that patient data could be directly entered by investigation sites into an EDC system when patients completed site visits. Thus, trial data could be collected in a timely fashion and avoid potential transcription errors. However, the real data collection practice at the site level is not as initially expected. Study coordinators often record the data on hospital charts (or flag notes or printed CRF pages) and then enter the data into the clinical EDC later. Obviously, this practice could potentially generate more transcription errors. Because of this, clinical trial monitors must conduct Source Data Verification (SDV) for the data entered into the clinical database (EDC). The current practice in most clinical trials is to conduct 100% SDV before database lock. This is a lengthy, tedious and costly procedure. The current data collection process is illustrated in the following picture.
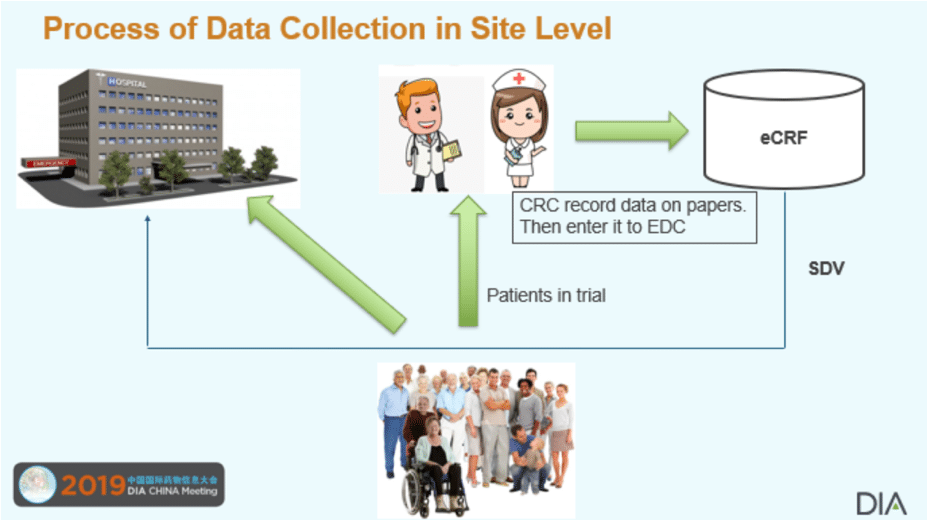
On the other hand, patient medical data such as lab tests are usually stored in the hospital system called Electronic Medical Record (EMR) or Electronic Health Record (EHR). Every day, health care professionals are updating patients’ electronic health records with data on clinical outcomes resulting from medical interventions used in routine clinical practice. Hopefully, the rich medical information can be used for supporting clinical research.
In July 2018, the FDA released guidance on the use of EHR data for clinical trials. This guidance is intended to assist sponsors, clinical investigators, contract research organizations, institutional review boards (IRBs), and other interested parties on the use of electronic health record data in FDA-regulated clinical investigations. The guidance’s goals are to modernize and streamline clinical investigations through the use of EHR data and the inclusion of real-world data in clinical investigations. In addition, the guidance will encourage sponsors and health care organizations to work with EHR and electronic data capture (EDC) system vendors to further advance the interoperability and integration of EHR and EDC systems.
Directly transmitting EMR/EHR into EDC would be an ideal way for collecting data for clinical trials. However, there are some barriers for direct transmitting data between EMR/EHR and EDC. The first is that the data structure and format are different. EMR/EHR uses Health Level-7 (HL7) standard whereas clinical database may use CDISC standard. The second is that hospitals may be not willing to open a channel in EMR/EHR for EDC to import the EMR/EHR data directly. With the encouragement of the FDA Guidance and advanced technologies nowadays, these barriers will be soon overcome.
In Dr. Xie’s talk, he will discuss his understanding of the FDA’s guidance, opportunities stimulated by the guidance, and the challenges with implementing the guidance.
DIA China
The 11th DIA China Annual Meeting 2019 is the largest event in the life sciences industry designed to foster the international exchange of actionable insights to improve health globally through the advancement of lifesaving medicines and technologies in the APAC region.
The DIA China Annual Meeting 2019 will bring together 3000+ pharmaceutical R&D professionals from different continents and regions, involved at all levels of the health care product development spectrum, to discuss recent and upcoming transformational changes for China’s innovation and regulatory environments. DIA China 2019 boasts more than 120 exhibiting companies, over 14+ themes, more than 80 sessions and explorable networking opportunities.
Dr. Tai Xie, CEO, CIMS Global
Tai Xie, Ph.D., is the founder and CEO of two companies: CIMS Global and Brightech International. He has over 24 years of clinical trial experience in pharmaceutical and CRO business. Dr. Xie is an expert in clinical trial design, clinical data management, eClinical Solutions and statistical analysis. He leads a team of over 100 people composed by statisticians, data analysts and software developers to provide services and eClinical Solutions to the pharmaceutical industry He is a member of American Statistical Association, Drug Information Association, ASCO, Sino-America Statistical Association and is an active journal reviewer for statistical journals.


